When it comes to oral care products targeting biofilm, understanding the regulatory considerations is essential. Biofilm is a complex microbial community that can form on the surfaces within the mouth, contributing to oral health issues such as gingivitis. This article delves into the regulatory landscape surrounding biofilm-targeted oral care products and their impact on gingivitis.
The Science of Biofilm and Its Relationship with Gingivitis
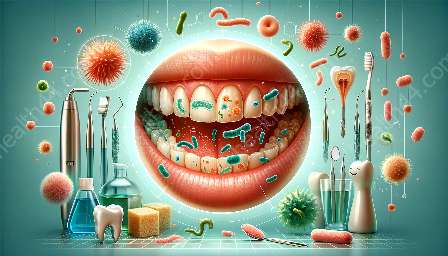
Biofilm is a structured community of microorganisms that adhere to surfaces and are embedded in a self-produced extracellular polymeric substance. In the oral cavity, biofilm can form on the teeth, gums, and other oral surfaces, leading to the development of oral diseases such as gingivitis.
Gingivitis is a common and mild form of gum disease characterized by red, swollen gums that bleed easily. It is primarily caused by the accumulation of plaque, a type of biofilm, on the teeth and gum line. The presence of biofilm in the oral cavity is a significant factor in the development and progression of gingivitis.
Regulatory Landscape for Biofilm-Targeted Oral Care Products
Developing oral care products that specifically target biofilm requires adherence to regulatory standards and guidelines. Regulatory authorities, such as the Food and Drug Administration (FDA) in the United States and the European Medicines Agency (EMA) in the European Union, have established frameworks for evaluating the safety and efficacy of oral care products, including those targeting biofilm.
Manufacturers of biofilm-targeted oral care products must conduct thorough preclinical and clinical studies to demonstrate the safety and effectiveness of their products. This often involves testing the products for their ability to disrupt or remove biofilm, reduce inflammation associated with gingivitis, and improve overall oral hygiene.
Key Regulatory Considerations
- Efficacy and Safety Testing: Products targeting biofilm must undergo rigorous testing to verify their ability to eliminate or control biofilm formation while ensuring safety for oral use.
- Labeling and Claims: Regulatory bodies closely scrutinize the labeling and claims of oral care products to ensure that they accurately communicate the intended use and benefits of the product without misleading consumers.
- Compliance with Good Manufacturing Practices (GMP): Manufacturers must adhere to GMP standards to ensure the quality, safety, and consistency of biofilm-targeted oral care products.
- Post-Market Surveillance: Regulatory requirements may include ongoing monitoring of product performance and safety once the product is available to consumers.
Compatibility of Biofilm-Targeted Oral Care Products with Gingivitis
Given the relationship between biofilm and gingivitis, the development of biofilm-targeted oral care products holds potential for managing and preventing gingivitis. These products are designed to disrupt the formation of biofilm and inhibit the growth of pathogenic bacteria that contribute to gingivitis.
By targeting biofilm, these oral care products aim to reduce the inflammation of the gums, promote healing, and improve overall oral health. Additionally, the use of biofilm-targeted products can complement standard oral hygiene practices, such as regular brushing and flossing, in the prevention and management of gingivitis.
Conclusion
Regulatory considerations play a crucial role in the development and marketing of biofilm-targeted oral care products, especially in their relevance to addressing gingivitis. Understanding the regulatory landscape and ensuring compliance with established standards is essential for manufacturers seeking to introduce innovative oral care solutions. As the science of biofilm and its impact on oral health continue to be studied, regulatory frameworks will evolve to support the development of safe and effective biofilm-targeted oral care products.